Search
- Page Path
- HOME > Search
Review Article
- Thyroid
- Active Surveillance for Low-Risk Thyroid Cancers: A Review of Current Practice Guidelines
- Min Joo Kim, Jae Hoon Moon, Eun Kyung Lee, Young Shin Song, Kyong Yeun Jung, Ji Ye Lee, Ji-hoon Kim, Kyungsik Kim, Sue K. Park, Young Joo Park
- Endocrinol Metab. 2024;39(1):47-60. Published online February 15, 2024
- DOI: https://doi.org/10.3803/EnM.2024.1937
- 2,444 View
- 180 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
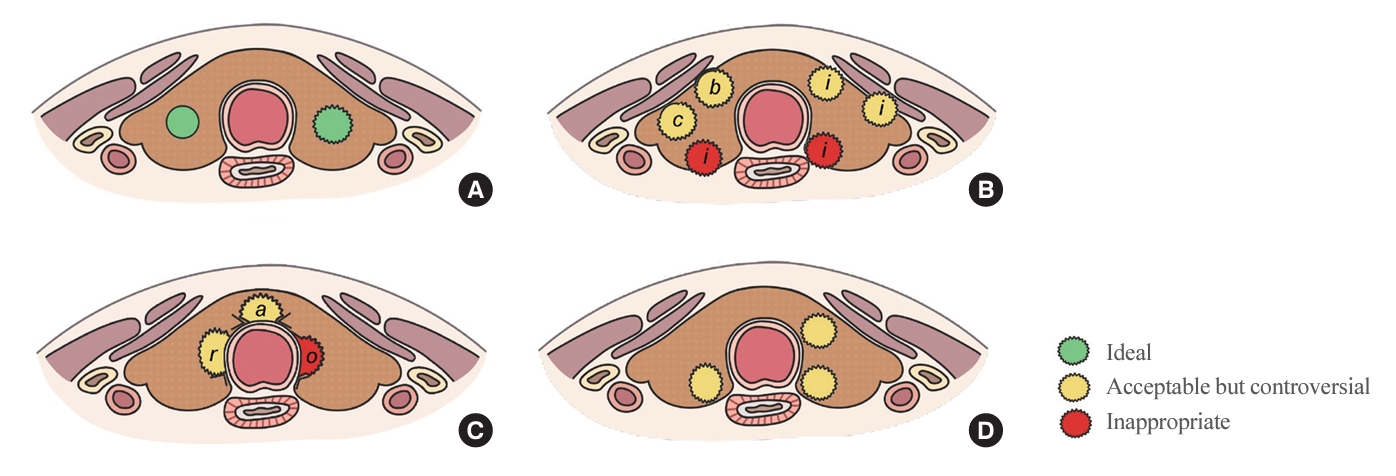
ePub - The indolent nature and favorable outcomes associated with papillary thyroid microcarcinoma have prompted numerous prospective studies on active surveillance (AS) and its adoption as an alternative to immediate surgery in managing low-risk thyroid cancer. This article reviews the current status of AS, as outlined in various international practice guidelines. AS is typically recommended for tumors that measure 1 cm or less in diameter and do not exhibit aggressive subtypes on cytology, extrathyroidal extension, lymph node metastasis, or distant metastasis. To determine the most appropriate candidates for AS, factors such as tumor size, location, multiplicity, and ultrasound findings are considered, along with patient characteristics like medical condition, age, and family history. Moreover, shared decision-making, which includes patient-reported outcomes such as quality of life and cost-effectiveness, is essential. During AS, patients undergo regular ultrasound examinations to monitor for signs of disease progression, including tumor growth, extrathyroidal extension, or lymph node metastasis. In conclusion, while AS is a feasible and reliable approach for managing lowrisk thyroid cancer, it requires careful patient selection, effective communication for shared decision-making, standardized follow-up protocols, and a clear definition of disease progression.
-
Citations
Citations to this article as recorded by- 2023 Update of the Korean Thyroid Association Guidelines for the Management of Thyroid Nodules
Eun Kyung Lee, Young Joo Park
Clinical Thyroidology®.2024; 36(4): 153. CrossRef
- 2023 Update of the Korean Thyroid Association Guidelines for the Management of Thyroid Nodules

Original Article
- Thyroid
Thyroid Cancer Screening - Diagnostic Performance of Ultrasound-Based Risk Stratification Systems for Thyroid Nodules: A Systematic Review and Meta-Analysis
- Leehi Joo, Min Kyoung Lee, Ji Ye Lee, Eun Ju Ha, Dong Gyu Na
- Endocrinol Metab. 2023;38(1):117-128. Published online February 27, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1670

- 2,246 View
- 167 Download
- 3 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
This study investigated the diagnostic performance of biopsy criteria in four society ultrasonography risk stratification systems (RSSs) for thyroid nodules, including the 2021 Korean (K)-Thyroid Imaging Reporting and Data System (TIRADS).
Methods
The Ovid-MEDLINE, Embase, Cochrane, and KoreaMed databases were searched and a manual search was conducted to identify original articles investigating the diagnostic performance of biopsy criteria for thyroid nodules (≥1 cm) in four widely used society RSSs.
Results
Eleven articles were included. The pooled sensitivity and specificity were 82% (95% confidence interval [CI], 74% to 87%) and 60% (95% CI, 52% to 67%) for the American College of Radiology (ACR)-TIRADS, 89% (95% CI, 85% to 93%) and 34% (95% CI, 26% to 42%) for the American Thyroid Association (ATA) system, 88% (95% CI, 81% to 92%) and 42% (95% CI, 22% to 67%) for the European (EU)-TIRADS, and 96% (95% CI, 94% to 97%) and 21% (95% CI, 17% to 25%) for the 2016 K-TIRADS. The sensitivity and specificity were 76% (95% CI, 74% to 79%) and 50% (95% CI, 49% to 52%) for the 2021 K-TIRADS1.5 (1.5-cm size cut-off for intermediate-suspicion nodules). The pooled unnecessary biopsy rates of the ACR-TIRADS, ATA system, EU-TIRADS, and 2016 K-TIRADS were 41% (95% CI, 32% to 49%), 65% (95% CI, 56% to 74%), 68% (95% CI, 60% to 75%), and 79% (95% CI, 74% to 83%), respectively. The unnecessary biopsy rate was 50% (95% CI, 47% to 53%) for the 2021 K-TIRADS1.5.
Conclusion
The unnecessary biopsy rate of the 2021 K-TIRADS1.5 was substantially lower than that of the 2016 K-TIRADS and comparable to that of the ACR-TIRADS. The 2021 K-TIRADS may help reduce potential harm due to unnecessary biopsies. -
Citations
Citations to this article as recorded by- To Screen or Not to Screen?
Do Joon Park
Endocrinology and Metabolism.2023; 38(1): 69. CrossRef - The 2017 United States Preventive Services Task Force Recommendation for Thyroid Cancer Screening Is No Longer the Gold Standard
Ka Hee Yi
Endocrinology and Metabolism.2023; 38(1): 72. CrossRef - Thyroid Cancer Screening: How to Maximize Its Benefits and Minimize Its Harms
Jung Hwan Baek
Endocrinology and Metabolism.2023; 38(1): 75. CrossRef - 2023 Korean Thyroid Association Management Guidelines for Patients with Thyroid Nodules
Young Joo Park, Eun Kyung Lee, Young Shin Song, Soo Hwan Kang, Bon Seok Koo, Sun Wook Kim, Dong Gyu Na, Seung-Kuk Baek, So Won Oh, Min Kyoung Lee, Sang-Woo Lee, Young Ah Lee, Yong Sang Lee, Ji Ye Lee, Dong-Jun Lim, Leehi Joo, Yuh-Seog Jung, Chan Kwon Jung
International Journal of Thyroidology.2023; 16(1): 1. CrossRef - Evaluation of the Appropriateness of Thyroid Fine-Needle Aspiration
Lairce Cristina Ribeiro Brito, Iara Beatriz De Carvalho Botêlho, Lanna Matos Silva Fernandes, Nayze Lucena Sangreman Aldeman, Uziel Nunes Silva
International Journal for Innovation Education and Research.2023; 11(6): 8. CrossRef
- To Screen or Not to Screen?


 KES
KES

 First
First Prev
Prev



